Development of Photosensitizers for Photodynamic Therapy
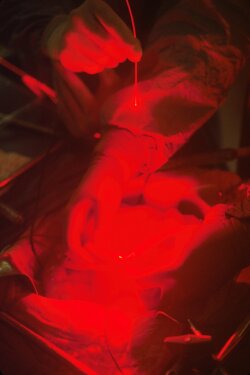
Following a surgical procedure, patients with tumors are typically further treated by chemotherapy. Despite the enormous success achieved with this technique, the patients undergoing these types of treatments experience severe side effects associated with the poor selectivity of the therapeutic agent for the cancer cells. To overcome this drawback, photodynamic therapy represents a promising alternative. During this medicinal treatment, the patient is injected with a non-toxic dose of a photosensitizer. After a certain incubation time, the targeted tissue is exposed to harmless laser irradiation. A therapeutic effect is only observed in the tissue, which contains the photosensitizer and is also exposed to the light source, resulting in precise and localized treatment (Figure 1).
Figure 1. Photograph of a surgeon treating a patient with photodynamic therapy.
Despite its clinical success, the photophysical and biological properties of the clinically applied photosensitizers are not ideal. Since the majority of these compounds are based on a tetrapyrrolic structural core (i.e., porphyrin, chlorin, bacteriochlorin, phthalocyanine), which strongly determines their photophysical and biological properties, these compounds are associated with a certain set of drawbacks including (1) poor water solubility, (2) poor photostability, (3) tedious synthesis/purification, (4) slow body clearance causing photosensitivity, and (5) poor cancer selectivity, resulting in the need of high drug and light doses for adequate tumor treatment.
To overcome these limitations, in our research group, research efforts are devoted to the design, synthesis, and biological evaluation of Ru(II) polypyridine complexes as photosensitizers for photodynamic therapy. Ru(II) polypyridine complexes are generally associated with high water solubility, high chemical stability and photostability, intense luminescence, large Stokes shift, and high reactive oxygen species production, making these metal complexes ideal photosensitizers for photodynamic therapy. Despite these remarkable properties, the majority of Ru(II) complexes are typically excited using blue or ultraviolet light and therefore suffer from a lack of absorption in the biological spectral window (600-900 nm). Based on absorption and light scattering effects in the biological environment, the light penetration depth into the tissue is low at this wavelength, which limits their application to treat deep tumors or large tumors. In an effort to circumvent this drawback, we have predicted the photophysical properties of new compounds with DFT calculations and rationally designed novel metal complexes with tailored photophysical properties (exemplary compound: Figure 2).
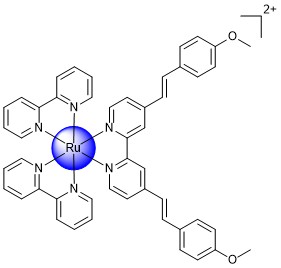
Figure 2. Structure of a Ru(II) complex as a photosensitizer for photodynamic therapy.
As predicted, the metal complex was found with excitation at longer wavelength in the biological spectral window. Using electron spin resonance spectroscopy and radical scavengers, the generation of singlet oxygen, a highly reactive and cytotoxic species, upon irradiation was identified. Importantly, the compounds were found to be highly water soluble, stable in organic solvents, water, and even human plasma as well as upon light irradiation, and therefore able to overcome the limitations of currently clinically applied PSs. Capitalizing on these impressive properties, the biological effects of these compounds were investigated. The complex was found to be internalized by an energy depended endocytosis pathway, where it primarily accumulated in the cytoplasm. Using a reactive oxygen-specific probe, the generation of reactive oxygen species in cancerous cells was verified by confocal microscopy. Importantly, no reactive oxygen species were generated in the dark but a strong production was observed upon exposure to irradiation (Figure 3). While being non-toxic in the dark, the compound demonstrated to be photoactive against cancer cells upon excitation from 480 nm up to clinically relevant 595 nm with cytotoxic values in the nanomolar range by triggering cell death through a combination of apoptosis and paraptosis pathways.
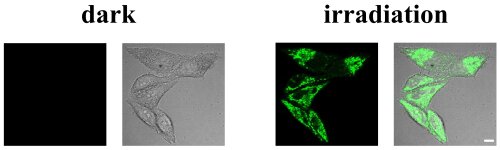
Figure 3. Confocal microscopy images of ovarian cancer cells incubated with Ru(II) complex und a reactive oxygen specific fluorescent probe.
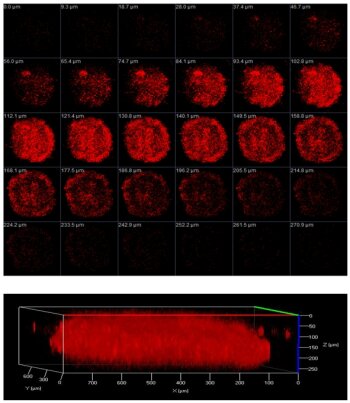
After evaluation of the effects of the compound on monolayer cells, its ability was investigated in multicellular tumor spheroids. This model can better simulate the conditions found in clinically treated tumors including hypoxia, proliferation gradients as well as simulate the drug delivery, which was found to be problematic for many investigated anticancer drug candidates. Using z-stack confocal microscopy, the full penetration of the three-dimensional architecture of the multicellular tumor spheroids was verified (Figure 4). Under identical conditions, the compound showed a more than two order of magnitudes stronger photodynamic effect in multicellular tumor spheroids than tetraphenylporphyrin, which was used a representative tetrapyrrolic photosenistizers.
Figure 4. Z-stack confocal microscopy images of an ovarian multicellular tumor spheroids incubated with the Ru(II) complex.
Overall, Ru(II) polypyridine complexes have attractive photophysical properties for an application as a photosensitizer. As the compound is highly water soluble and stable in human plasma as well as upon irradiation, this compound as well as structurally related derivatives are able to overcome all of the limitations of currently applied photosensitizers. Capitalizing on this, this class of compounds could show potential for clinical studies. Future studies are focused on the development of agents which could be activated at even longer wavelengths as well as remain phototoxic in hypoxic cancerous environments.
Key References
[1] J. Karges*, Clinical Development of Metal Complexes as Photosensitizers for Photodynamic Therapy of Cancer, Angew. Chem. Int. Ed. 2022, 61, e202112236. DOI: http://dx.doi.org/10.1002/anie.202112236
[2] J. Karges, S. Kuang, F. Maschietto, O. Blacque, I. Ciofini, H. Chao*, G. Gasser*, Rationally Designed Ruthenium Complexes for 1- and 2-Photon Photodynamic Therapy, Nat. Commun. 2020, 11, 3262. DOI: http://dx.doi.org/10.1038/s41467-020-16993-0
[3] J. Karges, F. Heinemann, M. Jakubaszek, F. Maschietto, C. Subecz, M. Dotou, R. Vinck, O. Blacque, M. Tharaud, B. Goud, E. V. Zahinos, B. Spingler*, I. Ciofini*, G. Gasser*, Rationally Designed Long-Wavelength Absorbing Ru(II) Polypyridyl Complexes as Photosensitizers for Photodynamic Therapy, J. Am. Chem. Soc. 2020, 142, 6578-6587. DOI: http://dx.doi.org/10.1021/jacs.9b13620