While traditional therapeutic methods (chemotherapy, radiotherapy, photodynamic therapy) rely on the generation of cytotoxic species, these therapeutic methods are generally associated with poor tumor selectivity and severe side effects. As an alternative method, much effort has been devoted to cancer immunotherapy in which the patient´s own immune system is activated or boosted to eradicate tumors. This allows for a more controlled and selective treatment of tumors. Despite promising results obtained with immunotherapy, cancerous tumors are able to form an immunosuppressive microenvironment which is rendering this treatment ineffective. To overcome this limitation, researchers are focusing on the development of agents that reprogram the immunosuppressive tumor microenvironment.
In our research group, research efforts are devoted to the design, synthesis, and biological evaluation of metal complexes as immune-activating agents. As one of the most promising strategies to enhance the immunotherapeutic effect, recent studies have indicated the activation of the stimulator of interferon genes (STING) pathway. Studies have shown two different distinguished mechanisms to inhibit the STING pathway: 1) DNA damaging agents, 2) STING protein binders. Despite preliminary promising findings, the use of currently studied compounds remains limited due to 1) poor accessibility and bioavailability, 2) poor DNA fragmentation, 3) poor water solubility and stability of the therapeutic agent, and 4) low cancer/tumor-targeting properties of the therapeutic agent. To overcome these drawbacks, in our group, a chemotherapeutic Pt(IV) prodrug, which could activate the STING pathway through DNA damage, was encapsulated with a PC7A-based polymer, which could activate the STING pathway through STING protein binding, was designed. For an effective therapeutic effect, the polymer backbone was designed with a reactive oxygen species sensitive linker and the terminal ends functionalized with polyethylene glycol for high water solubility (Figure 1).
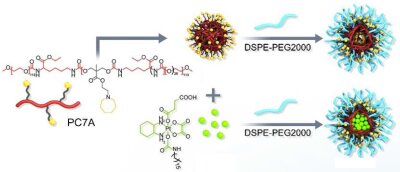
Figure 1. Formation of multimodal immune-activating nanoparticles of a chemotherapeutic Pt(IV) prodrug, which could activate the STING pathway through DNA damage, and a PC7A-based polymer, which could activate the STING pathway through STING protein binding.
The dual activation of the STING pathway was investigated by immunofluorescence confocal microscopy and Western Blot analysis. The results showed the phosphorylation and therefore activation of the STING protein as well as the overexpression of γ-H2A, as an indicator for DNA damage (Figure 2).
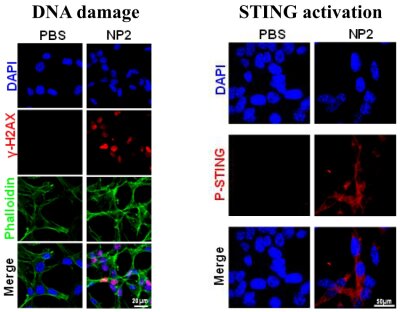
Figure 2. Immunofluorescence confocal microscopy of cancerous treated with the nanoparticles, causing a dual activation of the STING pathway through DNA damage and STING protein binding.
Overall, these findings indicate the potential of the STING pathway for cancer immunotherapy. Future studies are focused on the optimization of the pharmacological properties of the metal complex and the enhancement of the immunogenic response.
Key References
[1] X. Gao, G. Lei, B. Wang, Z. Deng, J. Karges*, H. Xiao*, D. Tan*, Encapsulation of Platinum Prodrugs into PC7A Polymeric Nanoparticles Combined with Immune Checkpoint Inhibitors for Therapeutically Enhanced Multimodal Chemotherapy and Immunotherapy by Activation of the STING Pathway, Adv. Sci. 2023, 10, 2205241. DOI: http://dx.doi.org/10.1002/advs.202205241
[2] L. Zhang#, N. Montesdeoca#, J. Karges*, H. Xiao*, Immunogenic Cell Death Inducing Metal Complexes for Cancer Therapy, Angew. Chem. Int. Ed. 2023, accepted. DOI: http://dx.doi.org/10.1002/anie.202300662
[3] D. Wei, Y. Huang, B. Wang, L. Ma, J. Karges*, H. Xiao*, Photo-Reduction with NIR Light of Nucleus Targeting Pt(IV) Nanoparticles for Combined Tumor-Targeted Chemotherapy and Photodynamic Immunotherapy, Angew. Chem. Int. Ed. 2022, 61, e202201486. DOI: http://dx.doi.org/10.1002/anie.202201486